Part:BBa_K3402050
report circuit
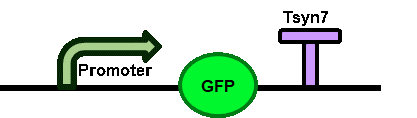
This device is composed of promoter Ptef1 (BBa_K3402007), reporter gene yeGFP (BBa_K3402000), terminator Tsyn7 (BBa_K3402001).
Usage and Biology
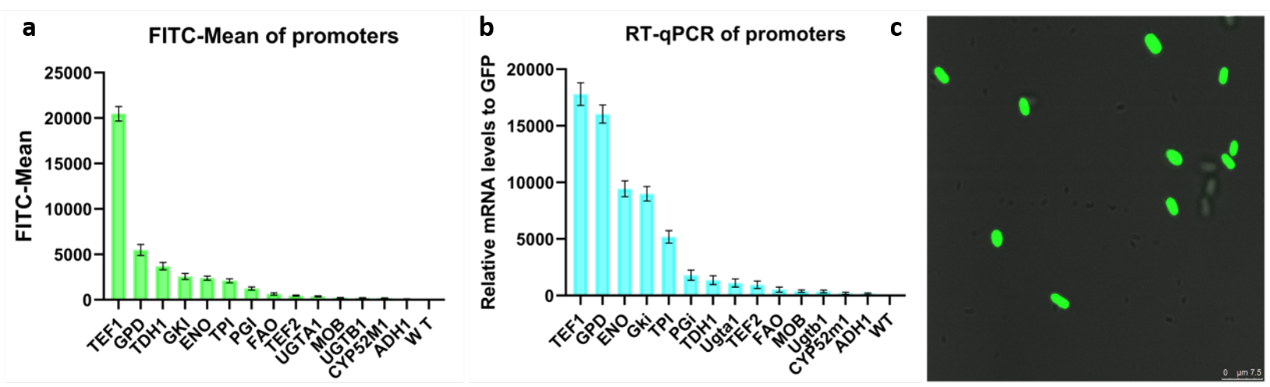
The green fluorescent protein (yeGFP) gene can be used as reporter gene and express in Starmerella bombocola. We conducted this device to test if the yeGFP can express in Starmerella bombocola, so we connected this device. Besides, the hygromycin resistance gene (hph) was built into the plasmid as the marker gene to determine if the transformation is successful. Two homologous arms are used to intsert this gene into PXA1 site.
As a result, the yeGFP was succefully expressed in Starmerella bombocola. Finally, we can link different promoters to yeGFP to know the strength of promoters by testing different fluorescence intensity and transcription levels analysis of different strains.
Improvement: IvyMaker-China 2021 iGEM Team
Our part BBa_K33829011 is a recombinant yeGFP improved from the part-reporter GFP BBa_K3402050 (iGEM20_Jiangnan_China). We optimized the codon and added a stronger promoter BBa_K3829001 and terminator BBa_K3829000. Besides, signal peptide (SS) BBa_K3829005 and V5-Tag BBa_K3829004 were involved.
Construction of plasmid P-SS-yeGFP3-V5-5105-T
In our project, yeGFP was used to screen anchored proteins.

Fig.1 Structure of P-SS-yeGFP3-V5-5105-T.
Through restriction enzyme digestion verification and sequencing, the plasmid was successfully constructed (Figure 2).

Fig.2 Verification of recombinant plasmids by restriction enzyme digestion. M: DL 15000 DNA Marker; 1:P-SS-yeGFP3-V5-5105-T double enzyme digestion (Xba Ⅰ & EcoR Ⅰ)
After the plasmid was successfully constructed, it was introduced into the target strain Candida tropicalis . And then the expression of yeGFP and the position of fluorescent was confirmed with CLSM (Figure 3).

Fig.3 Representative images of yeGFP (P-SS-yeGFP3-V5-5105-T) expression. The yeast morphology observed under the bright field (Left). The yeast morphology observed under green fluorescence excitation wavelength (Middle). Merged image (Right).
References
1.Eisenhaber, Birgit, et al. "A sensitive predictor for potential GPI lipid modification sites in fungal protein sequences and its application to genome-wide studies for Aspergillus nidulans, Candida albicans Neurospora crassa, Saccharomyces cerevisiae and Schizosaccharomyces pombe." Journal of molecular biology 337.2 (2004): 243-253.
2.Möller, Steffen, Michael DR Croning, and Rolf Apweiler. "Evaluation of methods for the prediction of membrane spanning regions." Bioinformatics 17.7 (2001): 646-653.
3.Smith MR, Khera E, Wen F. “Engineering Novel and Improved Biocatalysts by Cell Surface Display.” Ind Eng Chem Res, volume 53, issue 16, 29 April 2015, pp. 4021-4032.
4.Tanaka T, Yamada R, Ogino C, Kondo A. “Recent Developments in Yeast Cell Surface Display toward Extended Applications in Biotechnology.” Appl Microbiol Biotechnol, volume 75, issue 3, August 2012, pp. 577-591.
5.Andreu C, Del Olmo ML. “Yeast Arming Systems: pros and cons of different protein anchors and other elements required for display.” Appl Microbiol Biotechnol, volume 102, issue 6, Mar 2018, pp. 2543-2561.
Improvement: IvyMaker-China 2022 iGEM Team
Characterization- [Improvement]
This year, we replaced the promotor and terminator of GFP on the basis of last year. Promoter-FBA1 and Terminator-ADH2 would achieve better effect and orthogonality. And we used GFP and RFP as markers of successful construction of spycatcher and spytag syetem.
SP-GFP-His-Spytag+ SP-RFP-cMyc-Snooptag+ SP-CBM-SC-SC-SNC-SC-V5-7813The system is composed of three parts and we used different promotor and terminator for each part to achieve better orthogonality.
Part 1:SP-GFP-His-Spytag BBa_K4122014
Part 2:SP-RFP-cMyc-Snooptag BBa_K4122015
Part 3:SP-CBM-SC-SC-SNC-SC-V5-7813 BBa_K4122016
Characterization-Construction of Tag-Catcher systems.
To attain co-display, we combined our display system with two selective protein binding systems, SpyTag-SpyCatcher and SnoopTag-SnoopCatcher. In our experiment, GFP and RFP were used to indicate the successful construction of Spycatcher/Spytag and Snoopcatcher/Snooptag systems. We initially tried two catcher types with a ratio of 1:3.

Fig.1 The construction of plasmid Ts-PGAPDH--TENO1A, the surface display system for displaying both GFP and RFP. (BBa_K4122017)
SC: Spycatcher BBa_K4122008; SNC: Snoopcatcher BBa_K4122010; V5: V5 tag BBa_K3829004; CBM: carbohydrate binding domain BBa_K4122006
GFP+ and RFP+ suggested the successful construction of Spycatcher/Spytag system and Snoopcatcher/Snooptag system.

Fig.2 The fluorescence result of the spy/snoop tag and catcher system.
A and D, bright field; B and E, Green fluorescence; C and F, Red fluorescence
References
[1] Wei Zheng, Chengxin Zhang, Yang Li, Robin Pearce, Eric W. Bell, Yang Zhang. Folding non-homology proteins by coupling deep-learning contact maps with I-TASSER assembly simulations. Cell Reports Methods, 1: 100014 (2021).
[2] Chengxin Zhang, Peter L. Freddolino, and Yang Zhang. COFACTOR: improved protein function prediction by combining structure, sequence and protein-protein interaction information. Nucleic Acids Research, 45: W291-299 (2017).
[3] Jianyi Yang, Yang Zhang. I-TASSER server: new development for protein structure and function predictions, Nucleic Acids Research, 43: W174-W181, 2015.
[4] Lu, Hongyuan, et al. "Machine learning-aided engineering of hydrolases for PET depolymerization." Nature 604.7907 (2022): 662-667.
Sequence and Features| None |